PFAS Contamination in Water and its Removal with Reverse Osmosis Filtration
Per- and polyfluoroalkyl substances (PFAS) pose a growing threat to water sources globally. As regulatory bodies worldwide grapple with the challenges posed by PFAS, a spotlight is cast on the effectiveness of Reverse Osmosis Filtration in mitigating this crisis.
What are PFAS?
PFAS, or Per- and Polyfluoroalkyl Substances, are a group of human-made chemicals that feature strong carbon-fluorine bonds, which make them highly resistant to heat, water, and oil. These compounds have been widely utilized in various industrial and consumer products for decades due to their unique properties.
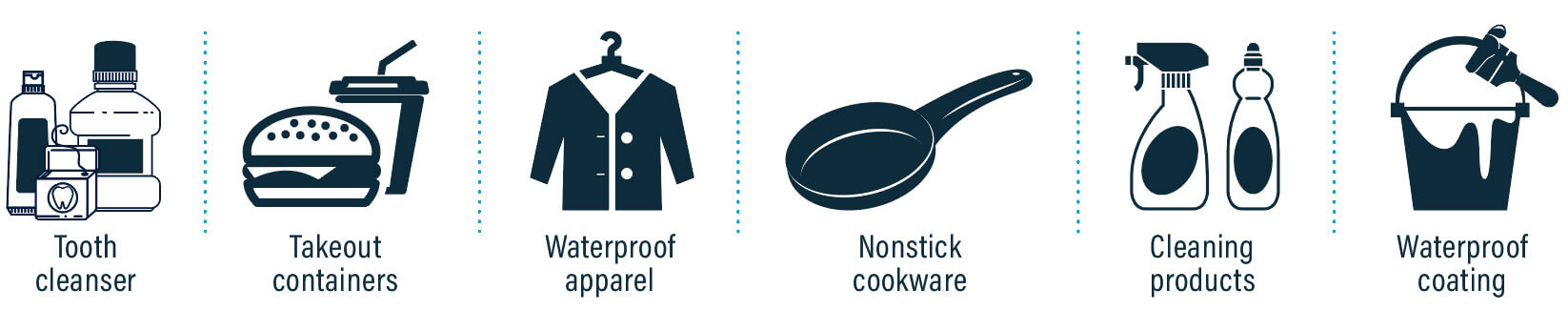
PFAS possess stable overall chemical properties, including resistance to water, oil, and high temperatures, making them challenging to decompose in the natural environment. Consequently, PFAS are referred to as "persistent chemical substances" and can accumulate in the environment, finding their way into drinking water supplies. These substances are commonly present in everyday items such as non-stick cookware, waterproof clothing, stain-resistant fabrics, food packaging, and cleaning products.
Potential Health Risks
According to data from the United States Environmental Protection Agency (EPA), there are nearly 15,000 members in the PFAS family. Among them, perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) are more easily absorbed by the environment and the human body due to their small molecular size and reactivity with surfaces.
The persistent nature of PFAS in the environment raises concerns about their potential health risks. The National Institutes of Health in the United States state that it takes an average of 3 to 7 years for PFAS to be metabolized in the body.
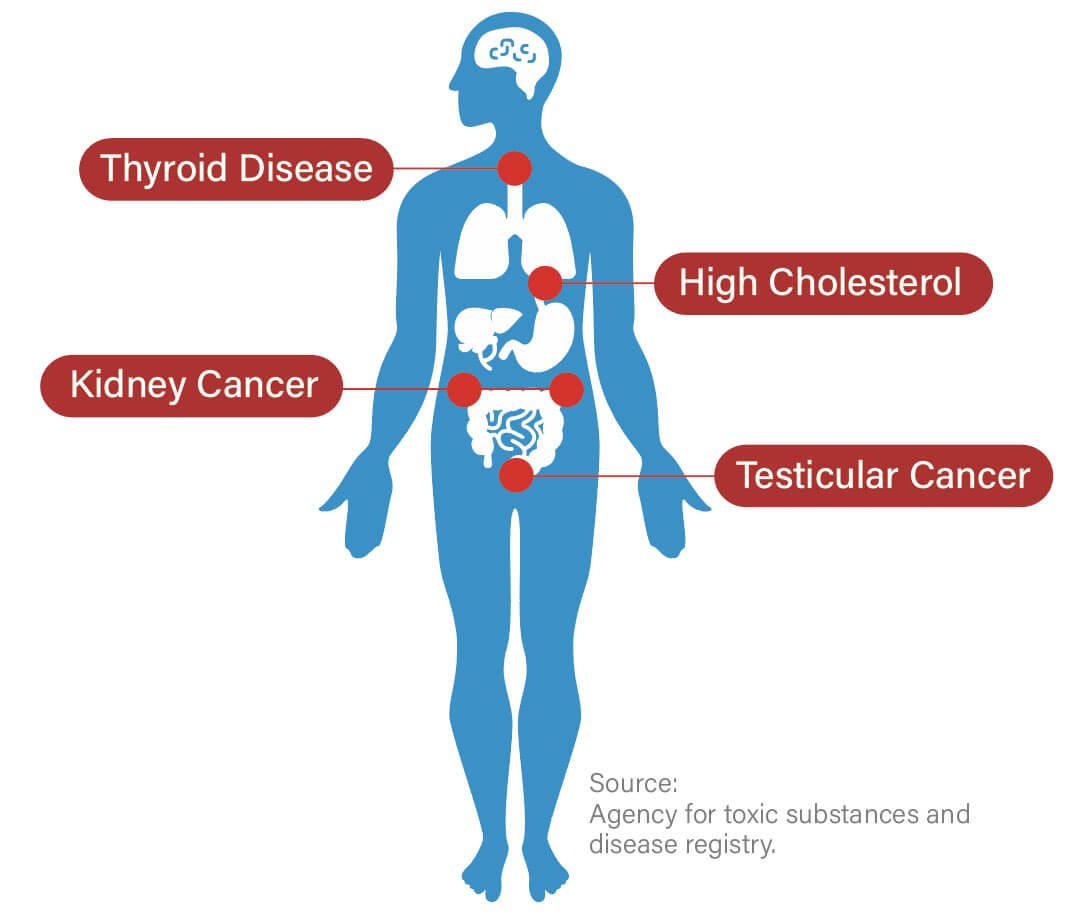
PFAS have been linked to a range of adverse health effects, including cancer, reproductive issues, and immune system and endocrine disruption, with more significant effects on the elderly, women, and children.
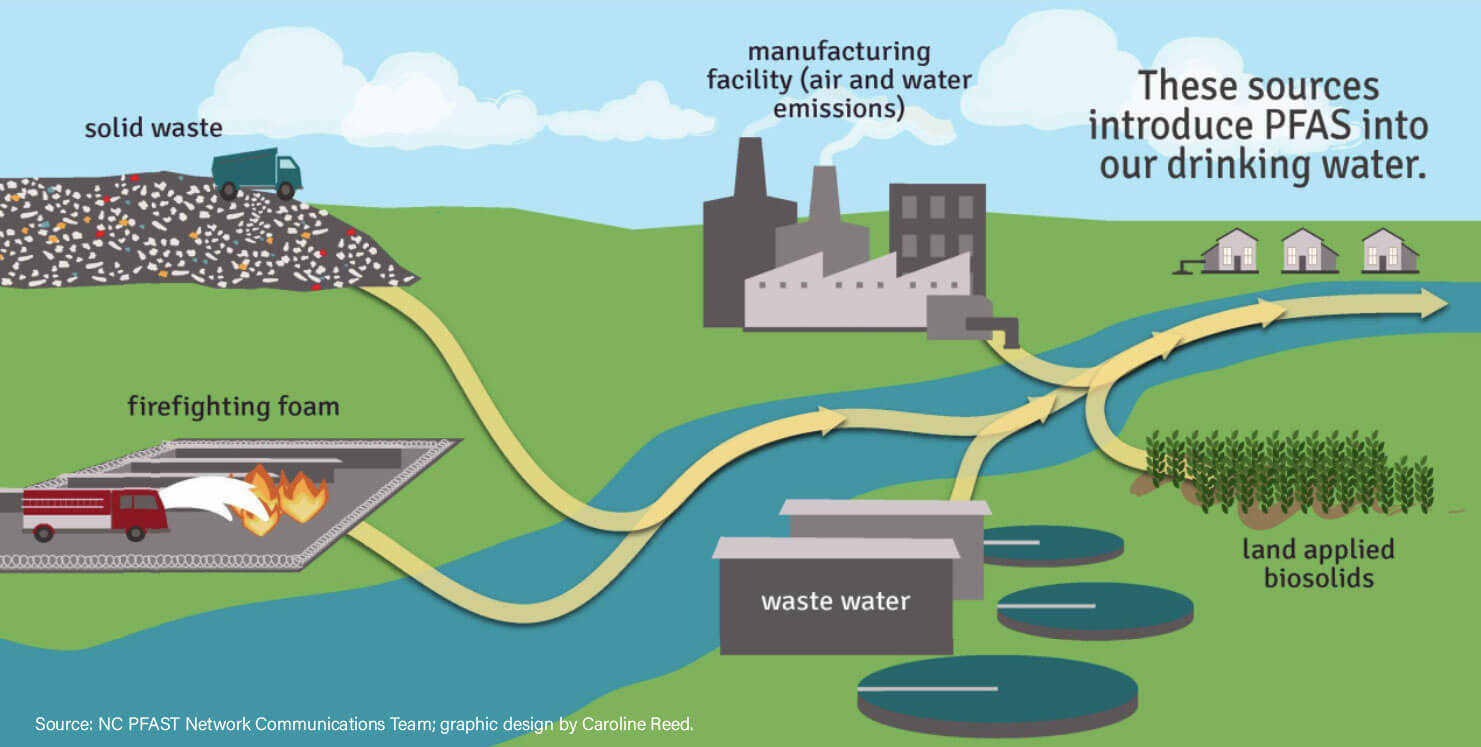
PFAS in Water Sources
One common source of PFAS exposure for the general public is through drinking contaminated water. PFAS can enter water sources through:
Manufacturing and Chemical Facilities
- PFAS are used in the production of a wide range of industrial and consumer goods, including textiles, electronics, and packaging materials.
- Industrial discharges from manufacturing plants can release PFAS into nearby water bodies, contaminating surface water and groundwater.
- Chemical facilities that produce PFAS-containing chemicals can release these substances into water sources through improper disposal practices.
Firefighting Foam
A significant source of PFAS contamination is firefighting foam, particularly aqueous film-forming foam (AFFF), used in emergency response situations at airports and military bases.
Landfills and Waste Disposal
Improper disposal of products containing PFAS can result in the leaching of PFAS into landfills. These contaminants can migrate into groundwater, affecting nearby water sources.
Airborne Transport
PFAS can be transported over long distances through the atmosphere. Once airborne, they may settle onto water bodies, contributing to contamination. This process is particularly relevant in regions where PFAS are produced or heavily used.
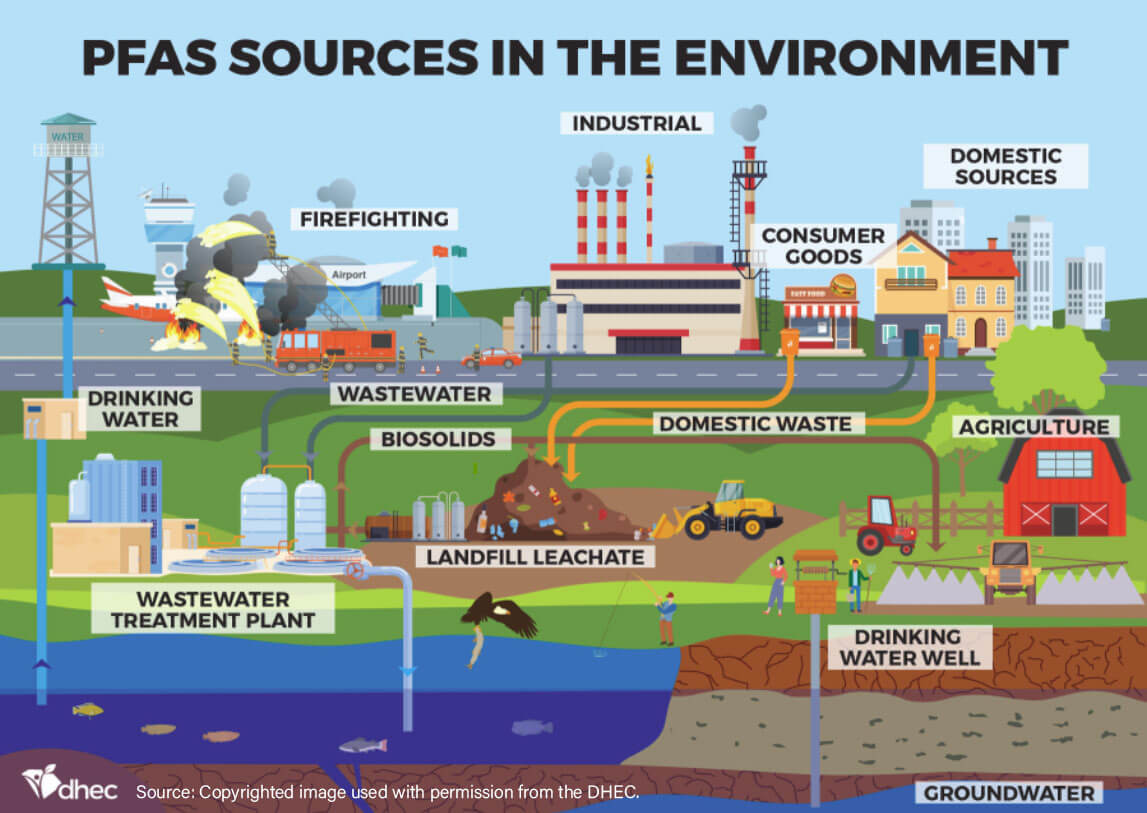
The Scope of PFAS Contamination
PFAS contamination has reached a global scale, impacting drinking water supplies and the environment in various regions.
-
Drinking Water Supplies
- Urban and Rural Areas: PFAS has been detected in drinking water supplies in both urban and rural areas.
- Public Water Systems: Many public water systems have reported elevated levels of PFAS, leading to concerns about the safety of tap water. This has prompted regulatory agencies to set guidelines and standards for PFAS levels in drinking water.
-
Environmental Impact
- Surface Water Contamination: PFAS can accumulate in rivers, lakes, and other surface water bodies. Aquatic ecosystems are at risk, impacting fish and wildlife through bioaccumulation and biomagnification within the food chain.
- Soil and Sediment Contamination: PFAS can bind to soil particles and settle in sediment, leading to long-term environmental contamination.
PFAS Regulations in Drinking Water
Several regulatory bodies have recognized the risks associated with PFAS contamination in drinking water and have established guidelines to protect public health. Here is an overview of existing regulations and key points:
-
United States - Environmental Protection Agency (EPA):
- Advisory Levels: The EPA has set a lifetime health advisory (HA) level for PFOS and PFOA at 70 parts per trillion (ppt) in drinking water. This advisory level is non-enforceable but serves as a reference for water system management.
- Monitoring and Reporting: The EPA recommends monitoring PFAS levels in drinking water and provides guidance on reporting results to regulatory authorities.
-
European Union (EU)
- Drinking Water Directive: The EU has established regulatory limits for PFAS in drinking water through its Drinking Water Directive. The directive sets a specific concentration limit for PFAS, and member states are required to monitor and control PFAS levels in their water supplies.
Key Regulatory Points
- Monitoring and Reporting: Regulatory bodies emphasize the importance of regular monitoring of PFAS levels in drinking water. Public water systems are required to conduct testing and report results to authorities.
- Health-Based Limits: Many regulations are based on health assessments, setting concentration limits for specific PFAS in drinking water to prevent adverse health effects.
- Guidance Values: Some guidelines provide non-binding guidance values, offering recommendations for managing PFAS contamination even if enforceable limits are not established.
Ensuring Safe Water through Effective Filtration
PFAS dissolve in water, and due to their chemical properties, traditional drinking water treatment technologies are unable to remove them. EPA researchers have studied various technologies to determine the most effective methods for removing PFAS from drinking water. These technologies include activated carbon adsorption, ion exchange resins, and membrane filtration, which encompasses nanofiltration and reverse osmosis (RO). These methods can be applied in various settings, ranging from large municipal water treatment facilities to residential filtration units used in homes. At the residential level, they can be employed at the point of entry, where water enters the home, or at the point of use, such as in a kitchen sink or shower.
Activated Carbon Treatment

Activated carbon is commonly used to adsorb natural organic compounds, taste and odor compounds, and synthetic organic chemicals in drinking water treatment systems. It is an effective adsorbent due to its highly porous nature, providing a large surface area for contaminants to adsorb. The EPA states that carbon filtration is 88-99% effective at treating certain PFAS.
Ion Exchange Treatment

Another treatment option is ion exchange treatment or resins. Ion exchange resins consist of highly porous, polymeric material that is acid, base, and water insoluble. The tiny beads composing the resin are made from hydrocarbons. Negatively charged ions of PFAS are attracted to the positively charged anion resins. Ion exchange has shown to have a high capacity for many PFAS; however, it is typically more expensive than activated carbon treatment. Like activated carbon, ion exchange removes nearly 100 percent of PFAS but only for a limited time, determined by the choice of resin, bed depth, flow rate, and the specific PFAS that need to be removed.
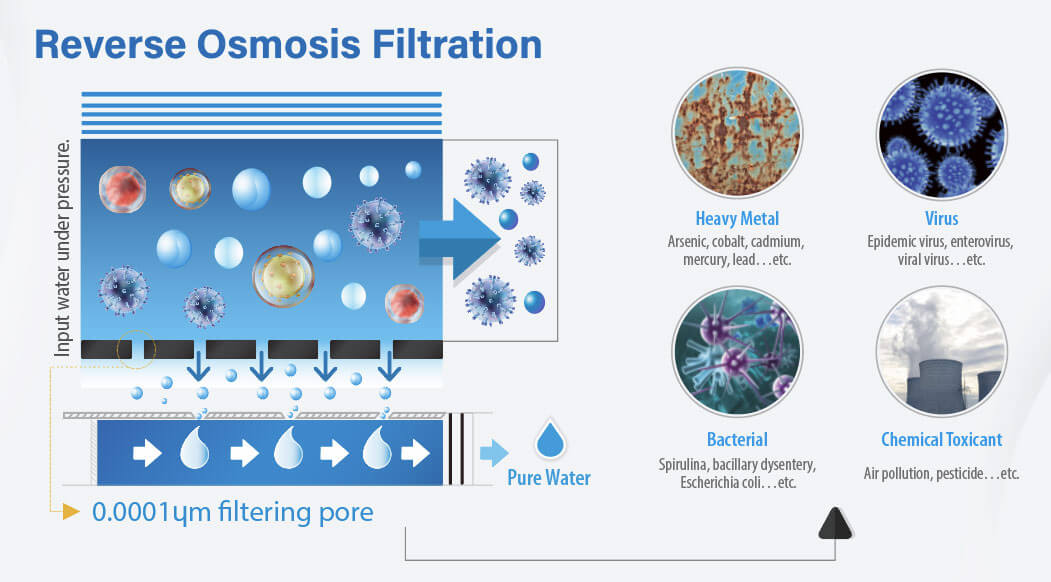
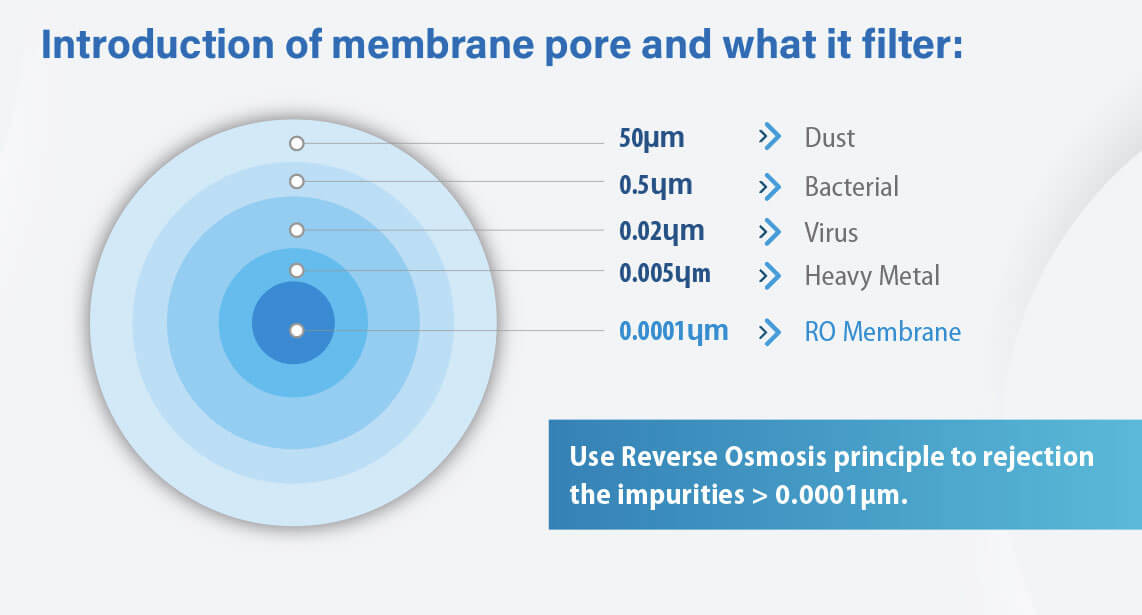
Reverse Osmosis Filtration

According to the EPA, reverse osmosis separation is up to 99% effective at removing certain PFAS. Unlike activated carbon or ion exchange, RO is not limited in its filtration capacity. Residential RO systems are generally used to treat water at the point of use (POU), such as for a specific faucet, and are easy to set up in a kitchen cabinet, usually below the sink. Besides PFAS, RO systems remove a greater range of contaminants than carbon or ion exchange systems.
The high efficacy of Reverse Osmosis Filtration in removing PFAS contaminants makes it an optimal choice for ensuring water safety. RO's versatility and lack of filtration capacity limitations make it highly economical over the long run for daily filtration of drinking water. Residential RO systems provide a practical solution, convenient for filtering at the point of use.